In Treatment-Naïve Patients,
Virologic Suppression Achieved at
Week 48 and Sustained to Week 96*
Study design
AMBER: Phase 3, randomized, double-blind, active-controlled, international, multicenter, noninferiority study assessing the efficacy and safety of SYMTUZA® (n=362) vs DRV/c + FTC/TDF (n=363) in treatment-naïve adults (n=725).† After Week 48, patients could continue on or switch to SYMTUZA® in an open-label, single-arm extension phase until Week 96.*
Primary endpoint: Proportion of patients with VL <50 copies/mL at 48 weeks (noninferiority margin 10% by FDA snapshot).1-3
Virologic response rates (FDA Snapshot)1
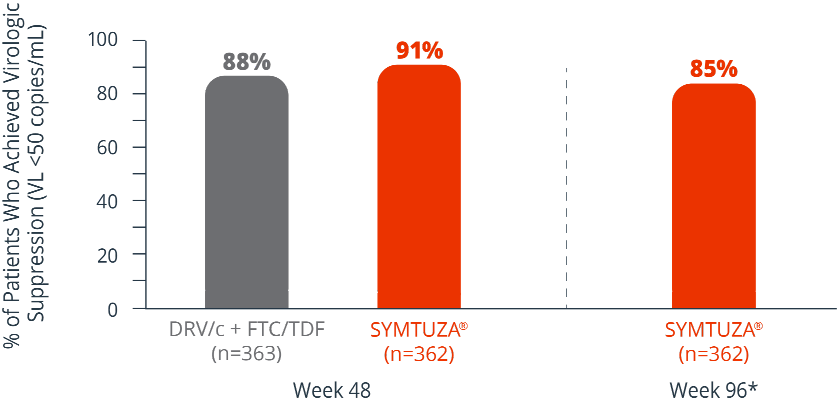
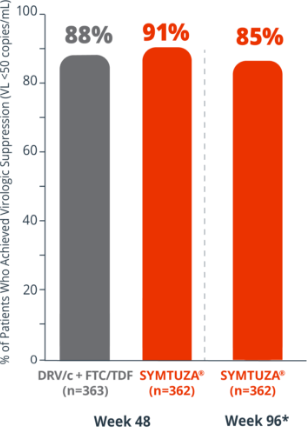
- 4% virologic failure rate (≥50 copies/mL) in the SYMTUZA® arm vs 3% in the DRV/c + FTC/TDF arm at 48 weeks1
- 6% virologic failure rate in the SYMTUZA® arm at 96 weeks*
- 4% of patients in the SYMTUZA® arm had no virologic data at 48 weeks vs 8% in the DRV/c + FTC/TDF arm1
- 9% of patients in the SYMTUZA® arm had no virologic data at 96 weeks*

treatment-emergent mutations in treatment-naïve patients1
*Week 96 was an open-label, single-arm extension, not a primary endpoint.1
†Randomization was stratified by VL and CD4+ cell count.3
DRV/c=darunavir/cobicistat; FTC=emtricitabine; TDF=tenofovir disoproxil fumarate; VL=viral load.
References: 1. Orkin C, Eron JJ, Rockstroh J, et al; AMBER Study Group. Week 96 results of a phase 3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS. 2020;34(5):707-718. 2. SYMTUZA® [package insert]. Titusville, NJ: Janssen Therapeutics, Division of Janssen Products, LP. 3. Eron JJ, Orkin C, Gallant J, et al; AMBER Study Group. A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS. 2018;32(11):1431-1442.
