Comparing ARV-Related Weight Gain in a Real-World Study
Real-World Data
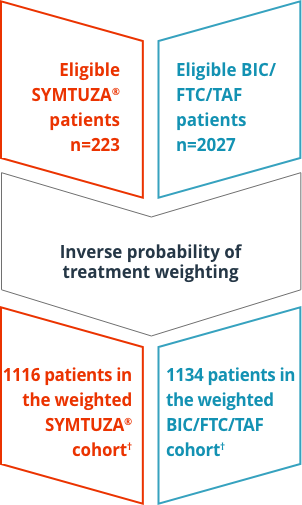
Study design
Retrospective, longitudinal study of EMR data on 2250 US patients with HIV-1 who initiated SYMTUZA® or BIC/FTC/TAF, collected between July 17, 2017, and March 1, 2020, from the Decision Resources Group Real-World Data Repository.* Study outcomes included:
- Mean change in BMI or weight between pre-index measurement and post-index measurements at 3-, 6-, 9-, and 12-months follow-up points
- Proportion of patients with a BMI or weight increase of >0%, ≥5%, and ≥10%
- Time to weight or BMI increase of ≥5% or ≥10% over 12 months

Inclusion criteria
- Newly initiated on SYMTUZA® or BIC/FTC/TAF (date of initiation defined as index date)
- Diagnosis of HIV-1 on or before the index date
- Either virologically suppressed (no viral load ≥50 copies/mL) for 6 months prior to index date or treatment-naïve
- ≥18 years of age
- ≥12 months of continuous clinical activity before the index date
- ≥1 BMI or weight measurement in both the baseline and follow-up periods
Exclusion criteria
- On ART but not virologically suppressed
- Diagnosis of HIV-2, liver disease (including cirrhosis or hepatitis), chronic renal insufficiency or creatinine clearance <30 mL/minute, pregnancy, or cancer‡ during the pre-index period
Study limitations
- Patients may not have adhered to treatment as prescribed
- IPTW only produces a valid causal estimate if all confounders are accounted for. Unmeasured factors may explain some observed changes in weight and BMI
- Patients without significant weight or BMI changes may not have undergone regular weight assessments. This analysis may only have captured more extreme weight changes
- The observed effect is assumed to be related to the whole regimen, rather than the specific components of the regimen. Further research is warranted to separate the effect of each component of these regimens on weight gain and BMI increase
- Patients who did not have evidence of HIV-1 viral load of ≥50 copies/mL or renal insufficiency (<30 mL/min/1.73 m2) were eligible for inclusion, but absence of evidence does not guarantee that the patient did not have either of these conditions
- This EMR data did not contain information on waist-to-hip ratio, and may have contained errors or omissions in variables of interest including diagnoses, weight values, BMI values, HIV-1 viral load measurements, and CD4+ count measurements
- Resistance information was not available in this data set. It was not possible to determine whether virologically suppressed patients had no known substitutions associated with resistance to components of the index regimen, as described in prescribing guidelines
Important note
This retrospective, longitudinal study of data from electronic medical records was designed to compare ARV-related weight gain in patients who were prescribed SYMTUZA® or BIC/FTC/TAF. It is important to note that this is one of many factors to consider when evaluating ARVs, and there are no head-to-head clinical trials comparing SYMTUZA® and BIC/FTC/TAF. HCPs should evaluate the risk-benefit profile of the individual drug products when making prescribing decisions for individual patients.
The results of this analysis should be viewed as hypothesis generating only. Results from this analysis reflect the findings of one study of real-world evidence and show similar results to other class-level real-world studies. Other real-world studies may show different results. Data presented in this section are not included in the Prescribing Information for SYMTUZA®.
Results
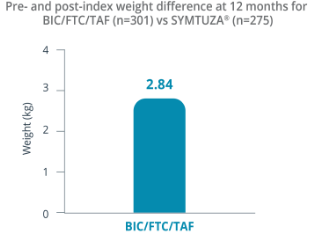
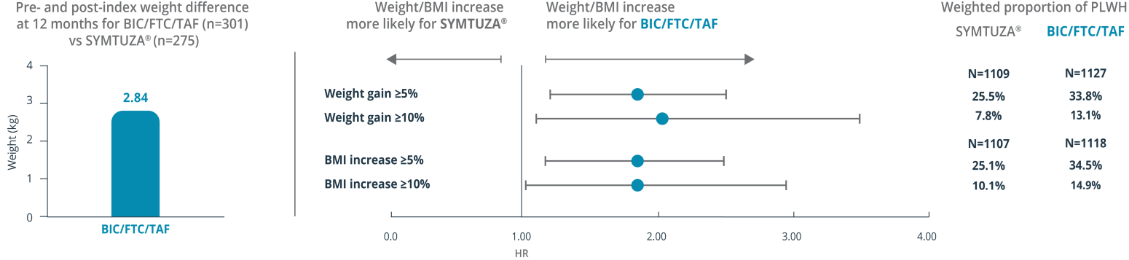
Adjusted Mean Difference in Weight§
Likelihood of Weight Gain by ARV Therapy Using Adjusted Hazards Ratios

*Part of Clarivate.
†Key baseline characteristics in weighted cohorts (SYMTUZA® [n=1116] vs BIC/FTC/TAF [n=1134]):
- Mean age (SD): 49.2 (+/- 12.1) vs 48.9 (+/- 12.8)
- Female: 27.9% vs 28.6%
- Race: 36.3% white & 35.5% Black/AA vs 34.4% white & 38.1% Black/AA
- Mean (SD) Quan-CCI score (not including HIV-1 symptoms) in both cohorts: 0.3 (+/- 0.6)
- Percentage living in the South: 72.9% vs 71.9%
- Percentage with commercial insurance: 67.9% vs 69.2%
In the weighted SYMTUZA® and BIC/FTC/TAF cohorts, 61.4% (n=685) and 60.3% (n=684), respectively, were previously treated with ART during the baseline period—of these patients, 50.1% and 49.3% used TAF. Among these ART experienced PLWHIV, 22% (151/685) and 25.6% (175/684) were still treated with their previous ART regimen in the last 45 days preceding the study index regimen. Among these patients, in the SYMTUZA® cohort, 33.1% were using a PI-based regimen, 57% were using an INSTI-based regimen, and 21.9% an NNRTI-based regimen. In the BIC/TAF/FTC cohort, 20.6% were using a PI-based regimen, 57.1% were using an INSTI-based regimen, and 28.6% were using an NNRTI-based regimen.
‡Excluding cutaneous Kaposi sarcoma, basal cell carcinoma, or resected, noninvasive cutaneous squamous carcinoma.
§Mean (SD) weight at pre-index for SYMTUZA® and BIC/FTC/TAF was 91.36 (±19.30) kg and 85.13 (±20.61) kg, respectively. Mean (SD) weight at 12 months post-index for SYMTUZA® and BIC/FTC/TAF was 90.07 (±19.70) kg and 87.28 (±20.98) kg, respectively.
AA=African American; ART=antiretroviral therapy; ARV=antiretroviral; BIC=bictegravir; BMI=body mass index; CCI=Charlson Comorbidity Index; EMR=electronic medical record; FTC=emtricitabine; INSTI=integrase strand transfer inhibitor; IPTW=inverse probability of treatment weighting; NNRTI=non-nucleoside reverse transcriptase inhibitor; PI=protease inhibitor; PLWHIV=people living with HIV; SD=standard deviation; TAF=tenofovir alafenamide.
Reference: Emond B, Rossi C, Côté-Sergent A, et al. Body mass index increase and weight gain among people living with HIV-1 initiated on single-tablet darunavir/cobicistat/emtricitabine/tenofovir alafenamide or bictegravir/emtricitabine/tenofovir alafenamide in the United States. Curr Med Res Opin. Published online December 7, 2021. 2022;38(2):287-298. doi:10.1080/03007995.2021.2007006
