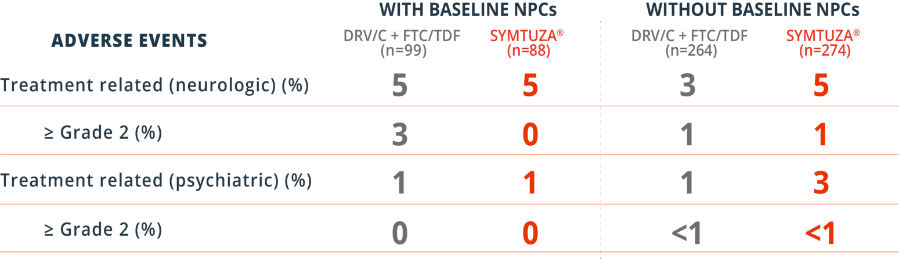
Consistent Tolerability Profile Regardless of Baseline Neuropsychiatric Comorbidities
Post hoc subgroup analysis of treatment-naïve patients through 48 weeks1*

additional risk of study discontinuation due to adverse events related to SYMTUZA®
in patients with baseline NPCs1†‡
*Among 725 patients, 88 (SYMTUZA®) and 99 (DRV/c + FTC/TDF) had baseline NPCs.1
In the SYMTUZA® arm, 43% (38/88) had neurologic comorbidities, such as headache (20%) or migraine (6%). In the DRV/c + FTC/TDF arm, 43% (43/99) had neurologic comorbidities, such as headache (21%) or migraine (8%).1
In the SYMTUZA® arm, 64% (56/88) had psychiatric comorbidities, such as depression (35%), anxiety (18%), or insomnia (7%). In the DRV/c + FTC/TDF arm, 70% (69/99) had psychiatric comorbidities, such as depression (27%), anxiety (14%), or insomnia (13%).1
†Compared to those without NPCs at baseline.1
‡This is a post hoc subgroup analysis that is not powered for safety or efficacy; statistical significance has not been established.1
ART=antiretroviral therapy; DHHS=Department of Health and Human Services; DRV/c=darunavir/cobicistat; FTC=emtricitabine; NPC=neurologic/psychiatric comorbidity; TDF=tenofovir disoproxil fumarate.
References: 1. Dunn K, Simonson RB, Luo D, et al. Use of D/C/F/TAF with neurologic/psychiatric comorbidities: AMBER subgroup analysis. Poster presented at: Conference on Retroviruses and Opportunistic Infections; March 8-11, 2020; Boston, MA. 2. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Updated January 20, 2022. Accessed March 8, 2022. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/whats-new-guidelines 3. Lowther K, Selman L, Harding R, Higginson IJ. Experience of persistent psychological symptoms and perceived stigma among people with HIV on antiretroviral therapy (ART): a systematic review. Int J Nurs Stud. 2014;51(8):1171-1189. 4. Fettiplace A, Stainsby C, Winston A, et al. Psychiatric symptoms in patients receiving dolutegravir. J Acquir Immune Defic Syndr. 2017;74(4):423-431.

