GI Adverse Events Decreased Over Time
In a post hoc analysis of treatment-naïve patients in AMBER (n=362), the majority of SYMTUZA® GI AEOIs* presented in Week 1 and decreased thereafter1
- The incidences of diarrhea and nausea were each 5% in Week 1, declining to ≤1% at Week 21
- All GI AEOIs were Grade 1 or 2 in severity and no serious adverse events were reported1
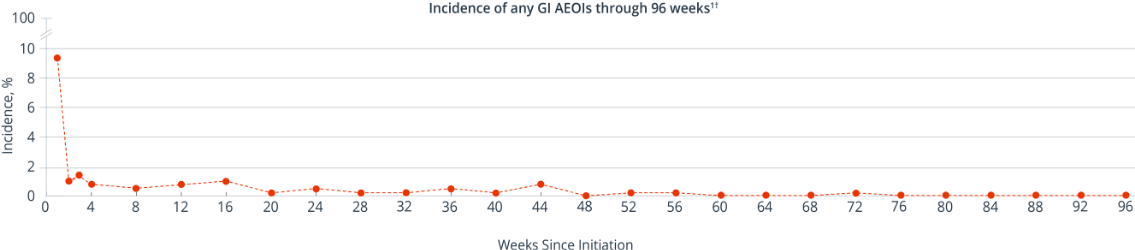
*A GI AEOI is defined as an adverse event that is related to the study drug. GI AEOIs were defined using the MedDRA preferred terms of diarrhea, nausea, abdominal discomfort, and flatulence.1
†Week 96 was an open-label, single-arm extension, not a primary endpoint.2
AEOI=adverse event of interest; GI=gastrointestinal; MedDRA=Medical Dictionary for Regulatory Activities.
References: 1. Dunn K, Baugh B, Bejou N, et al. Gastrointestinal (GI) adverse events with darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) through week 96: an AMBER post hoc analysis. Poster presented at: ID Week 2020 (virtual); October 21-25, 2020. 2. Orkin C, Eron JJ, Rockstroh J, et al; AMBER Study Group. Week 96 results of a phase 3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS. 2020;34(5):707-718.
